Top 3 Things You’ll Learn
- Four reasons why prescription drugs cost more in the U.S. than other countries
- Why consumers and leaders are looking to other countries for lower-cost drugs
- How importing drugs from Canada would impact the drug distribution system
Updated: Dec. 1, 2020
In late September, the U.S. Department of Health and Human Services (HHS) finalized a rule for states, Indian tribes, and potentially pharmacists and wholesalers to import certain drugs from Canada upon submitting a proposal to the FDA for review and receiving authorization to do so. The HHS specified that all eligible prescription drugs entering the U.S. would have to be “relabeled with the required U.S. labeling and undergo testing for authenticity, degradation and to ensure that the drugs meet established specifications and standards.” Several states, including Florida, have begun creating a drug importation plan seeking approval from the FDA.
The administration also finalized guidance enabling drug manufacturers “to voluntarily reimport their products authorized for sale in another country.” Currently, it is illegal for individuals to reimport prescription drugs intended for sale in Canada or other countries. Although insulin drugs are excluded from the program, the FDA finalized a guidance showing drug manufacturers “how to obtain a national drug code for certain FDA-approved biological products originally manufactured and intended for sale in foreign countries.” The Trump Administration did not disclose how much savings the program is anticipated to generate, nor did it comment on whether Canada agreed to the ruling.
Why Do Prescription Drugs Cost More in the U.S.?
It’s no secret that as the U.S. healthcare system moved from fixed copay plans to high deductible plans, consumers have had a tough time affording their medications. With the increasingly high cost of prescription drugs, importing drugs into the U.S. has become a popular idea among consumers, some state and federal legislators, and even the Trump Administration. However, there are multiple risks and concerns of this solution that are not being widely discussed. Understanding these concerns requires an understanding of why drugs cost more in the U.S. in the first place – and what’s at risk if we change our system to allow imported medications from foreign countries, like Canada.
There are four primary reasons that brand-name drugs cost more in the United States than anywhere else globally:
- Innovation – The majority of new drug discovery and research takes place in the United States for all of the world. Even drug manufacturers that have their importers located outside of the U.S. still have research laboratories in the U.S.
- Quality – The U.S. has the very highest standard of quality of medications, and part of that includes the inability to import drugs from outside the U.S. and a manufacturer that is not approved by the U.S. Food and Drug Administration (FDA). This focus on quality is one of the reasons we can go to bed at night knowing that our prescription drug supply is counterfeit-proof – unlike drugs that are imported from other countries.
- Access – The free market system in the U.S. is based on supply and demand. The U.S. does not ration access to medications as other countries do. For example, over the last few years, there has been 95 approved drugs to treat cancer globally. In the U.S., we have access to 95% of those drugs. In the United Kingdom, under a single payer health system, people have access to 49% of those drugs. Meanwhile in Greece, a relatively poor country with a single payer health system, people have access to just 9% of those cancer treatments. The single payer health systems we see across the globe are selective in the drugs that they allow, that it can be argued that they actually ration care in the use of essential medications.
- Subsidized – Like it or not, the U.S. subsidizes the price of drugs globally through its high cost of brand drugs. It may not seem fair, but the reality is that Americans pay more for branded drugs in the U.S. than people do elsewhere. A tremendous amount of dollars are spent on research. In fact, a 2020 study reports that it now takes $985 million to launch a new drug because for every one drug that is launched in the market, there are more than 900 drugs that never make it. A good chunk of that $985 million goes toward covering the cost of research and development of the drugs that never make it to market.
The U.S. healthcare system benefits from drug innovation, high quality standards, and access to life-saving medications, which come at a high cost. Many are looking to foreign drug markets as the answer, at the risk of causing a severe strain on supply and demand abroad.
Is Importing Drugs a Safe & Sustainable Solution?
We have a neighboring country – Canada – that operates as a sort of single payer health system, and it’s been well documented that prescription drugs in Canada cost less than drugs in the U.S. So why not just import drugs from Canada and other foreign markets?
The reality is that there are many potential safety risks that must be considered, including no system to monitor incidences of counterfeit or mislabeled drugs, potential health risks from a patient’s drug-drug interactions, and the possibility of people receiving expired or recalled drugs. In the case of counterfeits, you wouldn’t really realize that you aren’t getting the health benefit you anticipated. Because of their high price tags, cancer drugs have been a popular target for counterfeiters worldwide. Imagine you import what you think is a legitimate cancer treatment from a foreign country but later find out that drug was counterfeit; it would be a devastating blow.
There also are major economic concerns from a supply and demand perspective. The reality is that drug companies would not have any reason to increase the supply side at the equivalent rate as the demand side. So while some think this may be a good solution, what we would create is an unnecessary strain on Canada’s drug distribution system, resulting in massive drug shortages and rationing. This came to light on November 30, when Canadian Health Minister Patty Hajdu announced new measures aimed at protecting Canada’s drug supply. The new measures protect Canadians’ access to medication by prohibiting the bulk exportation of certain prescription drugs if doing so would cause or worsen a shortage.
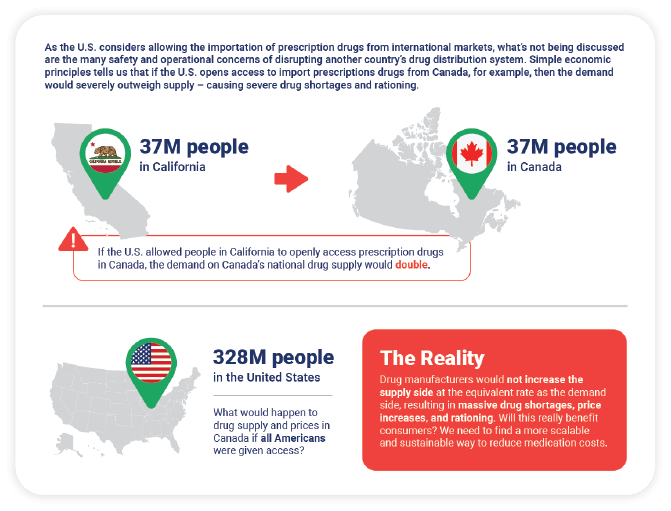
Consider this example: Canada has 37 million people and a drug distribution system that supports that many people. It doesn’t and couldn’t support 100 million or 200 million people. However, in the U.S., many state legislative bodies are passing laws that would allow the importation of drugs from Canada despite the fact that Canada has no interest in disrupting their drug distribution system to serve the demands of the U.S. If we consider the fact that California alone has 37 million people (the same number as Canada), and if we were to layer on other states like Florida, Texas, and New York, we would quadruple the demand on the Canadian distribution system.
This graphic shows that the reality of importing drugs from Canada is not so simple, nor is it a sustainable solution to address the high cost of drugs in the U.S.
Watch our on-demand webinar, Reality Check, to learn more about this and other hot topics impacting prescription drug costs and pharmacy benefit plan spending.
SOURCES
- The Hill: US Drug Prices Higher Than In The Rest of The World, Here’s Why
- JAMA: Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018
- Wall Street Journal: Counterfeit Cancer Medicines Multiply
- Fierce Healthcare: HHS, FDA Finalize Rule to Enable States to Reimport Cheaper Drugs from Canada