Top 3 Things You’ll Learn
- How generics are impacting PrEP pricing
- The barriers facing potential PrEP patients
- The latest requirements and regulations
In 2021, to reduce the spread of HIV by encouraging the use of HIV PrEP, the federal government enacted the “Ready, Set, PrEP” initiative, requiring plans to supply PrEP to members without any member cost share. Around the same time, the FDA approved a generic to Truvada, one of the only two approved PrEP drugs on the market. While activists, government programs, and experts struggle to promote the use of HIV PrEP to stem the tide of the HIV epidemic, benefits advisors need to help plan sponsors understand how the latest developments in drug manufacturing and government regulations impact their members.
The push to make PrEP accessible
HIV PrEP refers to Pre-Exposure Prophylaxis to prevent HIV infection, using virus-killing medications before exposure to HIV. PrEP can reduce the chance of getting HIV from sex or injection drug use. The medicine is taken on a continual basis and should be used in combination with safe sex practices. PrEP is recommended for people who have a higher risk of contracting HIV.
In 2021, the US Departments of Labor, Health and Human Services, and Treasury clarified that regulatory coverage requirements for PrEP under the Affordable Care Act intended to include all support services associated with PrEP (not just the medications), and that plans should provide those services at zero dollar copayment as well.1 This means that in addition to covering PrEP medications at a zero-dollar copay, plans must also assess zero-dollar copayments for support services such as baseline and follow-up testing and monitoring and counseling as necessary.2
What’s behind the increased plan cost for HIV PrEP?
The increase in PrEP plan cost PMPM over the last 18 months is largely due to the use of Descovy, the second of the two approved PrEP drugs. The most expensive option for PrEP at this time has had a slow, steady increase since January of 2021. Since the time when the generic equivalent to Truvada, emtricitabine/tenofovir disoproxil fumarate – known as FTC/TDF – became available, use of brand-name Truvada has dropped to nearly negligible levels.
An advertising campaign promoting the clinical superiority of Descovy over Truvada produced positive results for the drug maker: The number of prescriptions for Descovy for RxBenefits’ active book of business exceeded the number of prescriptions for the generic FTC/TDF for the period of January through May of 2021, and since that time has lagged only slightly behind the FTC/TDF generic in the number of prescriptions in spite of the significantly higher cost.
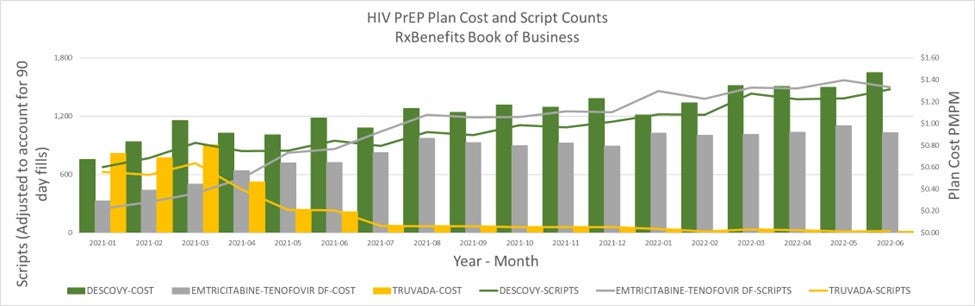
In addition to the relative increase in use of Descovy, recent cost increases for PrEP have also been driven by government and private efforts to increase PrEP utilization.
The one mitigating factor to these price-increasing forces is having a generic in the market, which has probably held the total cost of PrEP to a lower level than would have been seen without it.
Uptake of HIV PrEP is improving, but disparities exist
Use of PrEP by the 1.2 million people who should be taking it increased from about 3% in 2015 to about 25% in 2020,3 but barriers are numerous, including the stigma around HIV, misinformation, healthcare bias, and the complexity and cost of acquiring PrEP prescriptions.4 As a result, the number of people using PrEP remains lower than the national goal of 50% of those for whom PrEP is recommended.3
Though there are a wide range of interventions to address these barriers, PrEP uptake remains disappointing, made worse during the COVID-19 pandemic. Federal, state, and other support and advocacy groups are countering that low adoption with training, education, clinical guidelines, and focused outreach and awareness campaigns, and these types of programs are likely responsible for much of the improvement in adoption over the years. And it has had an impact – the HIV infection rate in the U.S. decreased by 9% between 2015 and 2019.
A new, long-acting injectable drug joins the market
But Truvada, Descovy, and associated generics no longer have the run of the market, thanks to FDA approval of Apretude at the end of 2021. Apretude – injected intramuscularly by a healthcare professional monthly for two months, then every two months thereafter – may increase adherence to the PrEP regimen because there is no need to remember to take a pill every day. However, the need to visit a healthcare facility and receive an injection will undoubtedly discourage some from using it.
Due to its administration requirements, Apretude is likely to be covered under the medical benefit for those plans choosing to cover it. Even though plans are required to cover just one product at zero-dollar copay, the segregation of pharmacy and medical benefit administration in many plans may mean that those plans will cover Apretude in addition to one or more of the oral preparations at no cost to the member.
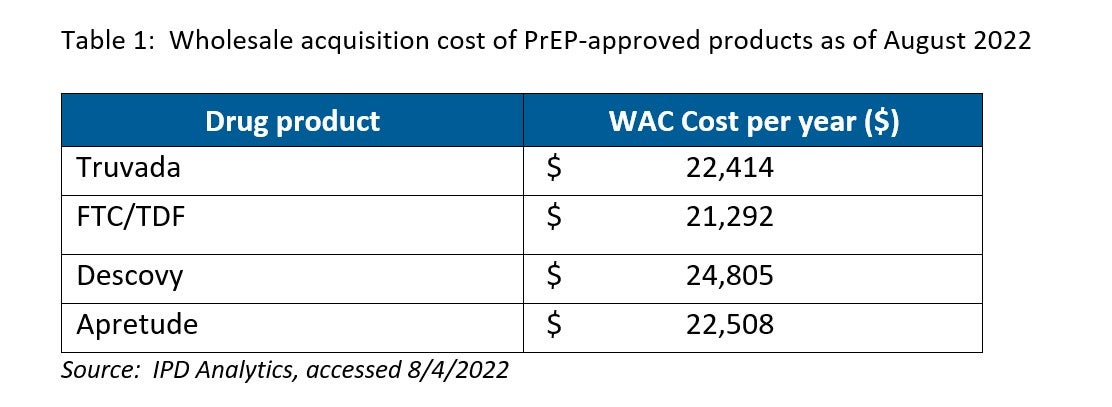
Commercial sources indicate that the annual cost for Apretude is actually less than the cost of Descovy and is similar to that of brand Truvada (see Table 1). Interestingly, generic FTC/TDF from most sources costs about $21,000 per year, but one source offers it for an annual cost of about $712.
As PrEP prescriptions continue to make an impact on HIV infection rates and drug costs, benefits advisors should keep their clients informed about the manufacturing and regulation developments impacting their plans. By shining light on these changes, and opportunities for patients, you can help in the fight against HIV.
Sources:
*Cost figures, unless otherwise noted, are drawn from RxBenefits data from its active book of business.
- FAQs About Affordable Care Act Implementation Part 47. Retrieved from dol.gov: https://www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-activities/resource-center/faqs/aca-part-47.pdf
- New Guidance On PrEP: Support Services Must Be Covered Without Cost-Sharing. Health Affairs, https://www.healthaffairs.org/do/10.1377/forefront.20210728.333084
- S. IS MAKING SUBSTANTIAL PROGRESS IN PREP FOR HIV PREVENTION. Retrieved from CDC.gov: https://www.cdc.gov/nchhstp/newsroom/fact-sheets/hiv/prep-for-hiv-prevention-in-the-us-factsheet.html#section2
- Barriers to Access for HIV PrEP. Retrieved from AJMC.com: https://www.ajmc.com/view/barriers-to-access-for-hiv-prep